

You can:
| Name | Muscarinic acetylcholine receptor M4 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CHRM4 |
| Synonym | HM3 M4 receptor cholinergic receptor Chrm-4 cholinergic receptor, muscarinic 4 |
| Disease | Produce mydriasis and cycloplegia for diagnostic purposes Hypertension Irritable bowel syndrome Moderate and severe psychomotor agitation Mydriasis diagnosis [ Show all ] |
| Length | 479 |
| Amino acid sequence | MANFTPVNGSSGNQSVRLVTSSSHNRYETVEMVFIATVTGSLSLVTVVGNILVMLSIKVNRQLQTVNNYFLFSLACADLIIGAFSMNLYTVYIIKGYWPLGAVVCDLWLALDYVVSNASVMNLLIISFDRYFCVTKPLTYPARRTTKMAGLMIAAAWVLSFVLWAPAILFWQFVVGKRTVPDNQCFIQFLSNPAVTFGTAIAAFYLPVVIMTVLYIHISLASRSRVHKHRPEGPKEKKAKTLAFLKSPLMKQSVKKPPPGEAAREELRNGKLEEAPPPALPPPPRPVADKDTSNESSSGSATQNTKERPATELSTTEATTPAMPAPPLQPRALNPASRWSKIQIVTKQTGNECVTAIEIVPATPAGMRPAANVARKFASIARNQVRKKRQMAARERKVTRTIFAILLAFILTWTPYNVMVLVNTFCQSCIPDTVWSIGYWLCYVNSTINPACYALCNATFKKTFRHLLLCQYRNIGTAR |
| UniProt | P08173 |
| Protein Data Bank | 5dsg |
| GPCR-HGmod model | P08173 |
| 3D structure model | This structure is from PDB ID 5dsg. |
| BioLiP | BL0339919,BL0339921, BL0339920 |
| Therapeutic Target Database | T20709, T50918 |
| ChEMBL | CHEMBL1821 |
| IUPHAR | 16 |
| DrugBank | BE0000405 |
| Name | carbachol |
|---|---|
| Molecular formula | C6H15ClN2O2 |
| IUPAC name | 2-carbamoyloxyethyl(trimethyl)azanium;chloride |
| Molecular weight | 182.648 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 1 |
| XlogP | None |
| Synonyms | D00524 ST51007309 8Y164V895Y DSSTox_RID_76703 WLN: ZVO2K1&1&1 &Q &G [ Show all ] |
| Inchi Key | AIXAANGOTKPUOY-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C6H14N2O2.ClH/c1-8(2,3)4-5-10-6(7)9;/h4-5H2,1-3H3,(H-,7,9);1H |
| PubChem CID | 5831 |
| ChEMBL | CHEMBL14 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | DB00411 |
Structure | 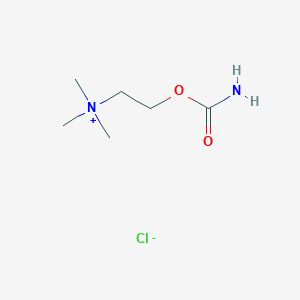 |
| Lipinski's druglikeness | Partition coefficient log P of this ligand is not available. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Activity | 50.0 % | PMID13678406 | ChEMBL |
| EC50 | 75.0 nM | PMID7783150 | ChEMBL |
| EC50 | 290.0 nM | PMID11741475 | ChEMBL |
| EC50 | 3981.07 nM | PMID17084634 | ChEMBL |
| EC50 | 60000.0 nM | PMID9651157 | ChEMBL |
| ED50 | 100.0 nM | PMID9651157 | ChEMBL |
| IC50 | 0.4 nM | PMID9873472 | ChEMBL |
| IC50 | 50.12 nM | PMID17149881 | ChEMBL |
| IC50 | 1000.0 nM | PMID13678406 | ChEMBL |
| IC50 | 7200.0 nM | PMID9435896 | ChEMBL |
| Inhibition | 6.3 % | PMID9651157 | ChEMBL |
| Inhibition | 60.0 % | Bioorg. Med. Chem. Lett., (1995) 5:6:631 | ChEMBL |
| Intrinsic activity | 1.0 - | PMID17084634 | ChEMBL |
| Ki | 7.6 nM | PMID12747793 | PDSP |
| Ki | 24.0 nM | PMID10891110 | ChEMBL |
| Ki | 398.11 nM | PMID17149881, PMID13678406 | ChEMBL |
| Ki | 1548.81 nM | PMID8968358 | PDSP |
| Ki | 2600.0 nM | PMID9622546, PMID10891110 | ChEMBL |
| Ki | 6309.57 nM | PMID19896386, PMID18543900, PMID16539379, PMID18077164, PMID24980056 | ChEMBL |
| Ki | 7600.0 nM | PMID12747793 | ChEMBL |
| pD2 | 5.43 - | PMID16539379, PMID17084634 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218