
You can:
| Name | Muscarinic acetylcholine receptor M3 |
|---|---|
| Species | Homo sapiens (Human) |
| Gene | CHRM3 |
| Synonym | cholinergic receptor cholinergic receptor, muscarinic 3 cholinergic receptor, muscarinic 3, cardiac Chrm-3 HM4 [ Show all ] |
| Disease | Urinary incontinence Overactive bladder Overactive bladder disorder Postoperative nausea and vomiting Respiratory disease [ Show all ] |
| Length | 590 |
| Amino acid sequence | MTLHNNSTTSPLFPNISSSWIHSPSDAGLPPGTVTHFGSYNVSRAAGNFSSPDGTTDDPLGGHTVWQVVFIAFLTGILALVTIIGNILVIVSFKVNKQLKTVNNYFLLSLACADLIIGVISMNLFTTYIIMNRWALGNLACDLWLAIDYVASNASVMNLLVISFDRYFSITRPLTYRAKRTTKRAGVMIGLAWVISFVLWAPAILFWQYFVGKRTVPPGECFIQFLSEPTITFGTAIAAFYMPVTIMTILYWRIYKETEKRTKELAGLQASGTEAETENFVHPTGSSRSCSSYELQQQSMKRSNRRKYGRCHFWFTTKSWKPSSEQMDQDHSSSDSWNNNDAAASLENSASSDEEDIGSETRAIYSIVLKLPGHSTILNSTKLPSSDNLQVPEEELGMVDLERKADKLQAQKSVDDGGSFPKSFSKLPIQLESAVDTAKTSDVNSSVGKSTATLPLSFKEATLAKRFALKTRSQITKRKRMSLVKEKKAAQTLSAILLAFIITWTPYNIMVLVNTFCDSCIPKTFWNLGYWLCYINSTVNPVCYALCNKTFRTTFKMLLLCQCDKKKRRKQQYQQRQSVIFHKRAPEQAL |
| UniProt | P20309 |
| Protein Data Bank | N/A |
| GPCR-HGmod model | N/A |
| 3D structure model | No available structures or models |
| BioLiP | N/A |
| Therapeutic Target Database | T67684 |
| ChEMBL | CHEMBL245 |
| IUPHAR | 15 |
| DrugBank | BE0000045 |
| Name | carbachol |
|---|---|
| Molecular formula | C6H15ClN2O2 |
| IUPAC name | 2-carbamoyloxyethyl(trimethyl)azanium;chloride |
| Molecular weight | 182.648 |
| Hydrogen bond acceptor | 3 |
| Hydrogen bond donor | 1 |
| XlogP | None |
| Synonyms | Choline, chloride carbamate(ester) 31325-EP2305672A1 Doryl AC1LAVH8 Ethanaminium, 2-((aminocarbonyl)oxy)-N,N,N-trimethyl-, chloride [ Show all ] |
| Inchi Key | AIXAANGOTKPUOY-UHFFFAOYSA-N |
| Inchi ID | InChI=1S/C6H14N2O2.ClH/c1-8(2,3)4-5-10-6(7)9;/h4-5H2,1-3H3,(H-,7,9);1H |
| PubChem CID | 5831 |
| ChEMBL | CHEMBL14 |
| IUPHAR | N/A |
| BindingDB | N/A |
| DrugBank | DB00411 |
Structure | 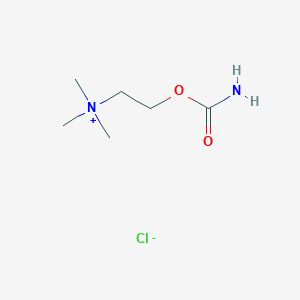 |
| Lipinski's druglikeness | Partition coefficient log P of this ligand is not available. |
| Parameter | Value | Reference | Database source |
|---|---|---|---|
| N/A | N/A | DrugBank | |
| Activity | 15.9 /s | PMID20716489 | ChEMBL |
| Activity | 350.0 % | PMID13678406 | ChEMBL |
| CCh | 100.0 % | Bioorg. Med. Chem. Lett., (1992) 2:8:821 | ChEMBL |
| EC50 | 70.0 nM | PMID3712371 | ChEMBL |
| EC50 | 1800.0 nM | PMID7783150 | ChEMBL |
| EC50 | 1819.7 nM | PMID17084634 | ChEMBL |
| EC50 | 1900.0 nM | PMID20716489 | ChEMBL |
| EC50 | 5011.87 nM | PMID17149881 | ChEMBL |
| EC50 | 10600.0 nM | Bioorg. Med. Chem. Lett., (1992) 2:8:821 | ChEMBL |
| EC50 | 15000.0 nM | PMID22329602 | ChEMBL |
| EC50 | 5.01187e+13 nM | PMID13678406 | ChEMBL |
| ED50 | 2.5 uM | PMID9651157 | ChEMBL |
| ED50 | 100.0 nM | PMID9651157 | ChEMBL |
| IC50 | 880.0 nM | PMID9873472 | ChEMBL |
| IC50 | 9100.0 nM | PMID9435896 | ChEMBL |
| Inhibition | 4.0 % | PMID10891110 | ChEMBL |
| Inhibition | 16.0 % | PMID9622546 | ChEMBL |
| Intrinsic activity | 1.0 - | PMID17084634 | ChEMBL |
| Ki | <10000.0 nM | PMID12235229 | PDSP |
| Ki | 20.0 nM | PMID12747793 | PDSP |
| Ki | 6309.57 nM | PMID17149881, PMID13678406 | ChEMBL |
| Ki | 20000.0 nM | PMID12747793 | ChEMBL |
| Ki | 43651.6 nM | PMID24980056, PMID18543900, PMID19896386, PMID16539379, PMID18077164 | ChEMBL |
| PI | 100.0 % | Bioorg. Med. Chem. Lett., (1995) 5:6:631 | ChEMBL |
| Stimulation | 100.0 % | PMID9651157 | ChEMBL |
| TIME | 1.742e-05 hr | PMID20716489 | ChEMBL |
zhanglab![]() zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218
zhanggroup.org | (734) 647-1549 | 100 Washtenaw Avenue, Ann Arbor, MI 48109-2218